Promotional Features
Umbrella effect for realigning hair and controlling frizz
Hair can vary in shape such as straight, wavy, or curly. These variations are related to the cross-section and internal bending angle of the hair fiber, which are genetically determined and programmed by the bulb, varying according to different ethnic groups, and can be classified into three large groups: Eastern, Caucasian, and African. 1-4
Regardless of the shape of the hair, the hair fiber is composed of approximately 80% of proteins and other elements such as lipids, water, and pigments, being formed by three parts: cuticle, cortex, and medulla. 4-6
The cuticle is the outermost part of the fiber and is the main barrier to the penetration of chemical agents into the inner part of the fiber; it is also responsible for the hair’s surface properties, such as shine. The medulla is located in the center of the fiber and its function is not yet fully understood, but its cells can create spaces filled with air and affect the hair’s shine. 4-6
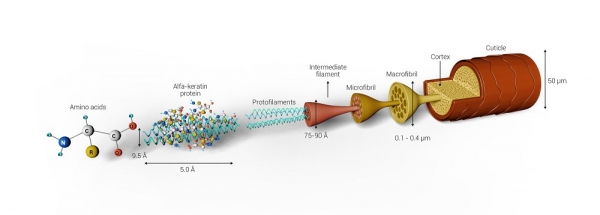
The cortex is the intermediate layer, where chemical transformations that result in straightening and curling take place; it constitutes the largest part of the hair fiber, being responsible for flexibility, elasticity, and physical strength. Its formation consists of alpha-keratin macrofibrils aligned in the direction of the thread and microfibrils, which in turn consist of intermediate filaments obtained from the interaction of alpha-keratin polypeptide chains, as shown in Figure 1. 4-6
Figure 1: Capillary structure formed by cuticle, cortex, and medulla. 4
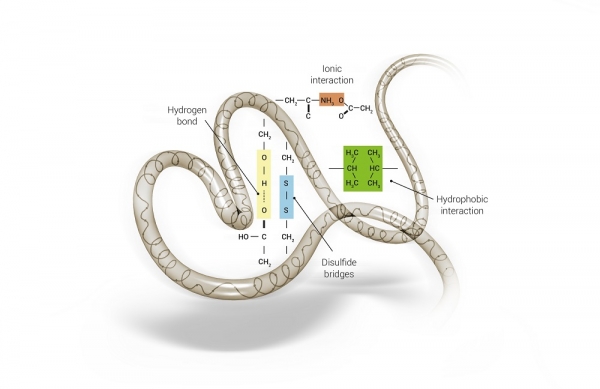
Alpha-keratin has a high content of disulfide bridges (S-S), from the amino acid cystine, which are the main bonds to maintain the strength and shape of the hair shaft. In addition to disulfide bridges, other intramolecular interactions contribute to the structural stability and mechanical strength of hair fibers, including hydrogen, hydrophobic and ionic or saline bonds, represented in Figure 2. 3,5,7
Figure 2: Chemical bonds present in the hair fiber (hydrophobic bonds, hydrogen bonds, ionic or salt bonds, and disulfide bonds). 4
To make the hair more aligned, and with less volume, it is necessary to increase the internal radius of curvature of the hair. This increase can be achieved by temporarily changing the conformation of the keratin structure from a-helix to β-sheet type, without breaking the disulfide bonds, a process known as temporary realignment. 3,5,7
With our goal to promote the temporary realignment of hair fibers, along with reducing hair volume and frizz, Chemyunion developed Allinea, a synergistic association of alpha-hydroxy acids, ethyl esters, disaccharide, coconut triglycerides, and the reuse of a biopolymer from a renewable plant-based source called lignin.
Action mechanism:
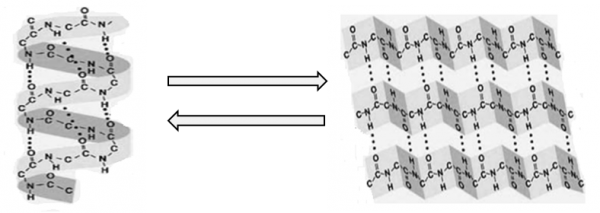
The alpha-hydroxy acids present in the composition of Allinea, associated with thermal energy, moisture, and physical action from brushing with a dryer and/or flat iron, favor the breaking of weaker chemical interactions such as hydrogen bonds, ionic and hydrophobic bonds. This temporary disruption leads to capillary relaxation, transforming the a-helix structure into a β-pleated sheet, increasing the internal bending angle of the hair fiber and repositioning new hydrogen bonds associated with alpha-hydroxy acids between the polypeptide chains, which can be reversed with the action of water as illustrated in Figure 3.7-9
Figure 3: Alpha-helix structure and a beta-pleated sheet of keratin.15 (Adapted from Staron et al., 2011).
In addition, Allinea contains trehalose, ethyl esters, coconut triglycerides, and lignosulfonate, a plant-based and lignin derivative biopolymer. The synergistic action of the ingredients creates protection for the keratin from protein denaturation, especially under desiccation and thermal stress conditions, by forming a hydrophobic film, reducing the action of water on the hair, and sealing the cuticles. 10-12
Composition and technical specifications:
Allinea is an active ingredient developed for the temporary realignment of hair fibers, that provides a reduction in hair volume and frizz. This is the result of a synergistic association of alpha-hydroxy acids, ethyl esters, disaccharide, smectite clay, coconut triglycerides, and the reuse of a biopolymer from a renewable plant-based source called lignin.
Efficiency:
Standard strands of hair were washed with shampoo and then treated with placebo leave-in, and one containing 5% Allinea, then brushed and straightened 10 times (220 °C). After the treatments, the strands were exposed to 70% relative humidity (RH) for 12 hours. Then volume and frizz measurements were performed before (T0) and immediately after treatments (PT) and after 2h (T2h), 6h (T6h) and 12h (T12h) of exposure, in three strands of hair per group (8 measurements per strand), through fiber orientation measurement using polarization imaging.
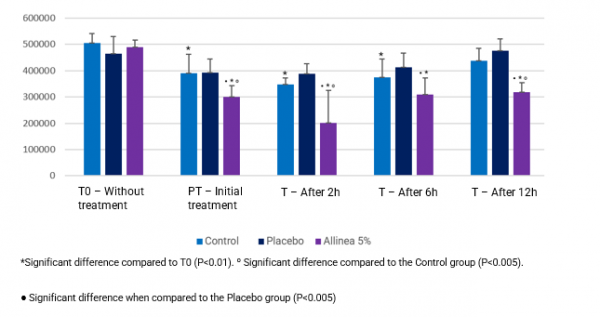
Results were evaluated by identifying the reduction in the total number of pixels – considering that the smaller the number of pixels the better Allinea’s performance of was in reducing hair volume. Allinea showed a significant reduction in volume when it came to strands of the Control groups (no treatment) after 2 and 12 hours of treatment; the Placebo group at all times evaluated; and after 2 hours of exposure, Allinea reduced hair volume by 58.6% when compared to T0 without treatment, as shown in Figure 4.
Figure 4: Evaluation of the volume of hair strands before treatment, after treatment with Allinea, and after exposure to high humidity (2h, 6h, and 12h).
Application:
Leave in, sprays, combing creams, and styling products.
Stable and compatible with cationic and non-ionic.
Indicated from 5%
------
References:
- JUNIOR, C.M. Estudo da formação, aplicação e do desempenho do tioglicolato de amino metilpropanol como um novo composto químico para tratamento de controle e redução de volume dos cabelos. Dissertação de Mestrado. Instituto de Pesquisas Tecnológicas do Estado de São Paulo – IPT. São Paulo. 2015.
- MIRANDA-VILELA, A.L., BOTELHO, A.J., MUEHLMANN, L.A. An overview of chemical straightening of human hair: technical aspects, potential risks to hair fiber and health and legal issues. International Journal of Cosmetic Science, 36, 2–11, 2014.
- VELASCO, M.V.R., DIAS, T.C.S., FREITAS, A.Z., JÚNIOR, N.D.V., OLIVEIRA PINTO, C.A.S.O., KANEKO, T.M., BABY, A. R. Hair fiber characteristics and methods to evaluate hair physical and mechanical properties. Brazilian Journal of Pharmaceutical Sciences vol. 45, n. 1, jan./mar., 2009.
- SANTOS, J. D. Caracterização de fios de cabelo antes e após tratamentos químicos e físicos por espectroscopias Raman e no infravermelho e microscopia eletrônica. Dissertação de Mestrado. Universidade Federal de Juiz de Fora. 2017.
- GOSHIYAMA, A.M. Avaliação das Propriedades das Fibras Capilares Tratadas com Alisante Ácido com Diferentes Valores de pH. Dissertação de Mestrado. Faculdade de Ciências Farmacêuticas. Universidade de São Paulo. 2019.
- LIMA, C.R.R.C. Caracterização Físico-química e analítica de fibras capilares e ingredientes cosméticos para proteção. Tese de Doutorado. Universidade de São Paulo. Faculdade de Ciências Farmacêuticas.2016.
- FERREIRA, L.A., BRAGA, D.C. Substâncias Ativas do Alisamento Capilar e Seus Mecanismos de Ação. Eletronic Jornal of Pharmacy, vol. XIII, n. 2, p. 56-63, 2016.
- ROBBINS, C.R. Chemical and Physical Behavior of Human Hair. Springer-Verlag Berlin Heidelberg, 5th Edition. 63-69, 205 -256, 671-677, 2012.
- STARON, P., BANACH, M., KOWALSKI, Z. Keratin – origins, properties, application. CHEMIK 65, 10, 1019-102, 2011.
- PYE, S. AND PAUL, P.K.C. Trehalose in hair care: Heat styling benefits at high humidity. J. Cosmet. Sci., 63, 233–241, July/August, 2012.
- ALMEIDA, A.M., CARDOSO, L.A., SANTOS, D.M., TORNÉ, J.M., FEVEREIRO, P.S. Trehalose and Its Applications in Plant Biotechnology.. In Vitro Cell.Dev.Biol. Plant. 43:167-177, 2007.
- OHTAKE, S., WANG, Y. J. Trehalose: Current Use and Future Application. Journal of Pharmaceutical Sciences, Vol. 100, No. 6, May, 2011.