Promotional Features
Tagra Biotechnologies: Improving cosmetic microencapsulation to create new user experiences
By Shaher Dochi, Ph.D. and Danny Goldstein. Ph.D.*
* Dannyg@tagra.com
Microencapsulation plays a vital role in the cosmetics industry. These technologies protect active ingredients, mask unpleasant odors and enable controlled release, making them key facilitators of the formulation, storage and application of cosmetic products. Yet, there remains a pressing need for improved microencapsulation technologies that overcome formulation challenges and enable novel user experiences.
All microencapsulation technologies seek to separate actives from forces of degradation, thereby stabilizing and maintaining the continuous benefits of these key ingredients. This separation is achieved through the use of shell materials that surround the actives, creating microcapsules of varying sizes that release the ingredient in response to mechanical action, temperature stimulation, diffusion, pH, biodegradation or dissolution.
However, while these core characteristics are common to all microencapsulation technologies, there are significant differences in the outcomes achieved by the various methods. Notably, some methods are incompatible with certain active ingredients or fail to protect them from degradation. These shortcomings result in stability problems.
Recognizing the problems, Tagra Biotechnologies tasked a team of PhD experts in encapsulation skin delivery systems with driving the sector forward, leading to the development of a unique microencapsulation process and issuance of 14 patents. The resulting microcapsules combine high loading, optimal protection, a release on demand mechanism and manufacturing tolerance to enable companies to overcome formulation challenges and add value to their products.
Why microencapsulation is hard
To develop a better, more broadly applicable microencapsulation technology, Tagra first had to understand the current landscape of products and their limitations.
There are three basic categories of microcapsules: matrix, monocore and polycore (Fig. 1). The matrix method disperses the active within the encapsulation materials. Monocore microcapsules feature a single chamber that houses the active. Polycore microcapsules have multiple chambers of different sizes. Formulators combine monocore and polycore structures to form new types of microcapsule.
Figure 1. Different types of microcapsules
The choice of shell material and microencapsulation method depends on the final application of the product and manufacturing costs.1-5 These choices dictate the morphology of the microparticle’s internal structure, which, in turn, influences the release of the active ingredient. A large number of other factors, such as the molecular weights of the polymer and active, also affect the release profile. This multitude of factors interact, further complicating the mechanism of release.
The complexity of this system of interacting forces is at the root of the two core, persistent problems facing companies that want to microencapsulate active ingredients. First, how do we design a microencapsulated system that will have specific desired drug release properties in vitro and in vivo? Second, even if we know exactly what is required, how do we manufacture it?
Tagra is leading efforts to find conclusive answers to these questions.
Tagra’s answer to formulation challenges
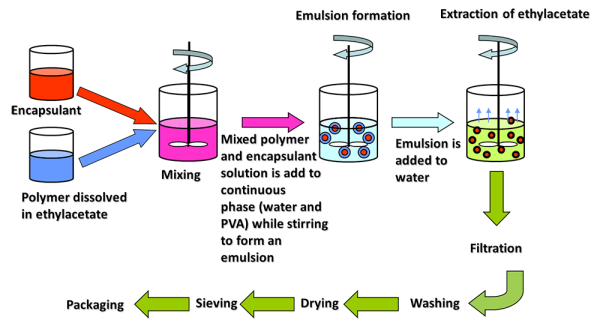
Tagra’s microencapsulation process is based on the solvent removal method. This facilitates the retention of lipophilic and water insoluble ingredients, such as actives, sunscreens and pigments, within the capsules over time (Fig. 2).
Figure 2. Illustration of Tagra’s production flowchart process.
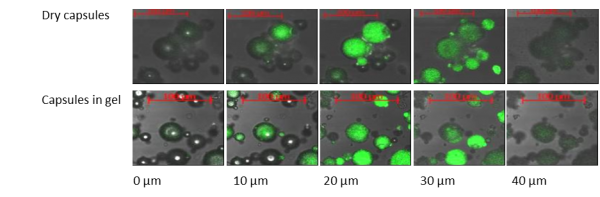
Tagra’s approach results in the creation of hermetic polymeric microcapsule walls with very tiny pores that stop actives from escaping and protects them from the detrimental effects of excipients including water (Fig. 3). The microcapsules appear in a powder form and do not break during formulation (Fig. 4).
Figure 3. Confocal microscopy images of Tagra microcapsules loaded with fluorescent prop demonstrate effective isolation of the active from the environment and the formulation both as dry powder or as microcapsules incorporated into gel system.
Figure 4. Tagra microcapsules of CelluCap A.
The microcapsules release their content by Tagra’s RND (Release on Demand) mechanism. This mechanism keeps the active encapsulated until the microcapsules are exposed to physical stress induced by applying the dosage form to the skin (Fig. 5). By simply pressing and rubbing during application, the polymeric walls collapse and the active ingredient is released onto the skin, leaving the unused formulation fully stable.
Figure 5. Tagra microcapsules Cameleon™ Yellow before (left) and after (right) application.
The core benefits of Tagra’s approach
Tagra’s approach to microencapsulation has some significant advantages. The microcapsules can hold high volumes of ingredients, withstand manufacturing processes such as pumping stress and protect actives from light, temperature, pH, oxidation and other materials, eliminating the stability issues that arise from using methods prone to degradation.
The approach is more versatile than the competition, too. Formulators can choose from a range of naturally-derived acrylic and/or cellulose polymers to encase their actives, reducing the risk of incompatibility between the encapsulant and other ingredients in the formulation. Overcoming the incompatibility problem eliminates another cause of stability problems.
While Tagra’s polymer shells are impermeable, they are also elastic. As such, the polymer shells rupture easily when exposed to mechanical pressure. This RND mechanism enables companies to create unique ingredient stories and user experiences. From the lab to the shelf, Tagra’s microcapsules help create successful beauty.6-8
How to use Tagra’s technology
Tagra has leveraged its core microencapsulation technology to create a range of products with specific features and benefits:
• Visual effects: Tagra’s vegan, naturally-derived microencapsulation pigments CameleonCaps create visual and sensory effects, including color and texture transitions, which engage the end consumer. Globally-approved encapsulated pigments are durable during formulation and scale up and break easily beneath fingertips upon application for dramatic and consistent color transformation.
By using CameleonCaps skin care is easily converted to color cosmetics, and color cosmetics become easy to produce with our free-flowing, dust-free powders. Spend less time color matching, less time with grinding and cleaning efforts and more time creating new trends.
Figure 6. Tagra’s shade chart helps guides the conversion of skin care into color cosmetics.
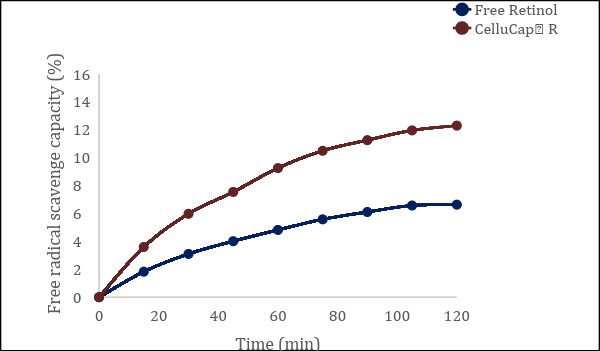
• Clinical results: Made from cellulose based polymers, CelluCaps use Tagra’s unique RND technology to create smart encapsulations for superior active delivery. Encapsulating lipophilic active agents results in a new physical form, a powder that can be dispersed and integrated across all phases of a formulation.
This creates a higher effective concentration of the active, as it is present both in oil phase and water phase. Upon application, while rubbing the formulation into skin, the polymeric shell collapses and the microcapsules begin to fracture, releasing the encapsulated active. CelluCaps give consumers confidence in active efficacy, and give formulators greater flexibility for creating stable, high-performance products for restoring skin.
Figure 7: CelluCapTM R shows improved anti-oxidant activity over unencapsulated Retinol.
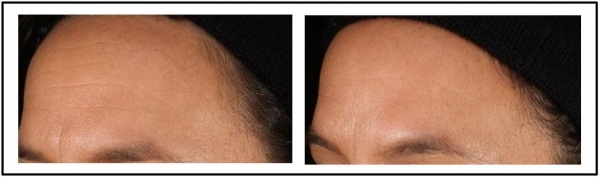
Figure 8: CelluCap R resulting in reduction of the appearance of forehead wrinkles [L] Base Line [R] 28 Days
• Enhanced stability and improved compatibility: Tagra’s encapsulated materials resist degradation, color change and odor shifts.
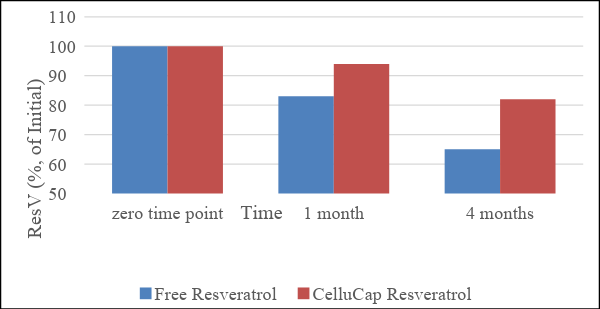
Figure 9: Stability of CelluCap Resveratrol microcapsules compared to free Resveratrol incorporated in topical O/W formulation after 1 and 4 months storage at 40 °C. Data are presented as mean % of initial content (n = 3).
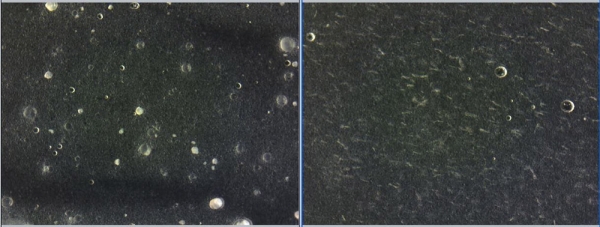
Figure 10: Light microscopy micrograph of formulations containing 4.25% CelluCap Resveratrol (left) or 0.6% free Resveratrol (right) following 4 months storage period at 25 C. Crystallization visible with free Resveratrol.
• Seamless scale up: Tagra’s encapsulations are unique in their ability to maintain integrity during production, and stay cohesive until application. Performance in the lab is what you see in manufacturing, with no special equipment required.
• Flexibility: Tagra’s encapsulations can be incorporated into any type of cosmetic formulation, in a vast array of combinations of color, sun care and actives. Tagra developed unbreakable transparent microcapsules, called the SunCaps family, to offer encapsulated organic and natural sunscreens.
• Expert R&D support: Partner with Tagra to create customized proprietary technologies that facilitate the development of value-added products and novel user experiences.
References:
1. G. Davidov-Pardo, D. J. McClements.2014. Resveratrol encapsulation: designing delivery systems to overcome solubility, stability and bioavailability issues. Trends in Food Science & Technology, 38: 88–103.
2. Y. O. Jeon, J. S. Lee, H. G. Lee.2016. Improving solubility, stability, and cellular uptake of resveratrol by nanoencapsulation with chitosan and γ-poly (glutamic acid). Colloids and Surfaces. B: Biointerfaces. 147: 224–233.
3. C. Hu, Q. Wang, C. Ma, Q. Xia. 2016. Non-aqueous self-double-emulsifying drug delivery system: A new approach to enhance resveratrol solubility for effective transdermal delivery. Colloids and Surfaces. A: Physicochemical and Engineering Aspects. 489: 360–369.
4. Casanova F, Santos L.2016. Encapsulation of cosmetic active ingredients for topical application – a review. Journal of Microencapsulation. 33:1-17.
5. Rama Dubey, T.C. Shami and K.U. Bhasker Rao.2009. Microencapsulation Technology and Applications. Defence Science Journal.59: 82-95.
6. Dochi S., Goldstein D. 2014. A Novel Microencapsulation Technology Ensuring Retinol Stability and Activity. SOFW journal. 140: 26-33.
7. Dochi S., Goldstein D. 2017. Microcapsules for effective skin lightening formulations. Euro Cosmetics: The International Magazine for Cosmetics and Fragrances. 25:18-21.
8. Müller S.2017. Current Sun Protection Innovations in Formulation and Application. SOFW journal. 3: 2-6.