Promotional Features
Human hair proteome profiling reveals the importance of KAPs
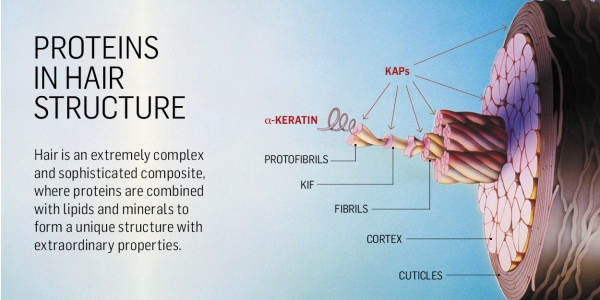
Hair is essentially composed of a complex arrangement of proteins, mainly keratins and keratin-associated proteins (KAPs), involving three subgroups of structures already well described: external cuticular layer, an inner cortex and, occasionally, a central medulla.
Different keratins intertwine to form protofibrils that, in turn, are organized into larger macrostructures called keratin, intermediate filaments or KIF further strengthening the structures known as fibrils.
The different polypeptide chains comprising the family of hard α‐keratin intermediate filaments result from specific gene expressions and two distinct classes of intermediate filament have been distinguished. These classes are known as class I (“acidic”) keratins K9–K10, K12– K28, and K31–K40 and class II (“basic to neutral”) keratins K1–K8 and K71–K86 keratins. The available evidence strongly supports an obligatory interaction between a type I and a type II chain to form the basic structural heterodimers of the keratin intermediate filament.
KIFs are present in the cortex and cuticle. In the cortex, keratins are embedded in an inter-filamentous matrix consisting of keratin-associated proteins, or KAPs, which are essential for the formation of a rigid and resistant hair shaft as a result of disulfide bonds between cysteine residues, among other interactions.
KAPs are the main components of the matrix. They are made up of small proteins which have different levels of cystine and glycine-tyrosine and can be classified into 3 groups: KAPs with high sulfur content (UHS, Ultra High Sulfur); KAPs with sulfur (HS, High Sulfur); Low sulfur or high glycine-sulfur KAPs (HGT, High Glycine-to-Tyrosine). HS KAPs dominate in the matrix.
Cuticles are cysteine and glycine rich, with the outer layers comprising highly cross-linked UHS-type proteins and the endocuticle mostly composed of low-sulfur residues and acidic and basic proteins. Cuticular KAPs are high in cysteine, serine, glycine and proline content, and cortex KAPs are low-sulfur structures, with a high concentration of basic and acidic residues when compared with cuticular proteins. They are considered to have originated independently and are essential for the formation of rigid and resistant hair shafts through their extensive disulfide bond cross-linking with the abundant cysteine residues of hair keratins or hydrophobic interactions with keratins (figure 1).
(Figure 1: Hair Diagram)These components form a strong and compact protein complex connected to a network of intra and intermolecular interactions, which are essential to promote mechanical resilience to the hair structure to environmental factors or chemical treatments.
There is, therefore, a clear interest in studying the complex interactions between hair proteins such as keratins and KAPs from quantitative, qualitative, and functional perspective.
Considering the various possibilities of this arrangement, which include genetic factors, compositional variations and alterations resulting from the exposome of each individual, the understanding of the structure-property relationship of the hair has only recently started to be elucidated thanks to the progress in hair proteomics.
Hair proteomics
According to Lyu, and collaborators (Essentials of Genomic and Personalized Medicine, Acad. Press, 2021), Proteomics is the analysis of the entire protein complement of a cell, tissue, or organism under a specific, defined set of conditions.
Created in 2001 as a by-product of the study of the human genome, proteomics is defined as a set of techniques used to explore the presence of proteins in a sample with the objective not only of understanding its composition but also to understand their interactions.
Proteomics relies on three basic technological breakthroughs: methods to fractionate complex proteins or peptide mixtures, advanced Mass Spectrometry methods to acquire the data necessary to identify individual proteins, and bioinformatics to analyze and assemble the data.
From the fundamental work of Sinclair and colleagues (The proteomic profile of hair damage, Brit. J. Dermatol. Vol. 166, p. 27-32, 2021), it became clear that the use of proteomic analysis was a valuable tool for analyzing hair shaft disorders and damage. Since then, proteomics techniques have been increasingly used in cosmetic science, especially in studies on the structure and properties of hair, opening new horizons for understanding the behaviour of different types of hair in different situations.
The works of J. M. Dyer et. Al. investigating the bleaching and alkali damage in human hair (Int. J. Cosm. Sci.,2013, 35 (6) p. 555-561, J. M. Marsh et al. on the role of Copper ions on the photochemical damage of hair (Int. J. Cosm. Sci, 2014, 36 (1), p.32-38 and A. J. Grovesnor et. Al on the physical and chemical disruption of human hair after bleaching (Int. J. Cosm. Sci, 2018, 40 (6), p.536) shed more light on these fascinating new tools.
The reduction of the presence of specific keratins and KAPs in aging hair was demonstrated by M. Giesen et al. (Experimental Dermatology, 2011, 20 (9), p. 759-761), point to the need of further studies on hair aging.
In all the studies carried out so far it has been demonstrated that KAPs, as the main group of proteins in the hair matrix, are directly affected by chronological aging or by exposure to chemical and physical agents, with a direct impact on the properties of the hair. It also known now that different KAPs are more sensitive to damage than others when exposed to such agents (figure 2).
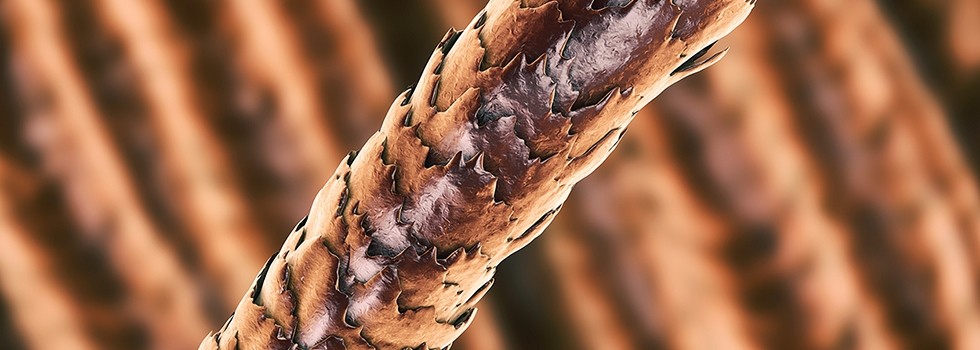
(Figure 2: Chemically damaged hair)The adoption of increasingly advanced proteomics techniques in the investigation of hair composition, structure and properties has greatly increased our understanding of the chemical structure of hair - from the existence of different keratins and their interactions in the formation of protofibrils to the knowledge about KAPs, a huge group of proteins essential for understanding the complex interactions in the hair fiber, will definitely help hair scientists to better understand the changes that occur in hair, whether caused by age, the environment or cosmetic treatments – as well as to create innovative solutions for hair care products.
Despite all the advances, it is still somewhat surprising that our standard thinking still associates hair conditions and properties only to keratin, ignoring the crucial role of protein-associated keratins – the KAPs – on hair care and health. They deserve much more of our attention.