The U.S. Food and Drug Administration has launched a new real-time reporting dashboard specifically for adverse events linked to cosmetic products. The platform offers manufacturers, suppliers, and other stakeholders a publicly accessible view of reported safety concerns as they are submitted.
Part of the agency’s FDA Adverse Event Reporting System (FAERS), the dashboard includes both mandatory serious adverse event reports required under the Modernization of Cosmetics Regulation Act of 2022 (MoCRA) and voluntary submissions from consumers, healthcare providers, salon professionals, and others.
The system is searchable and filterable by product name, adverse event term, severity, date, and report type. Reports are updated daily, allowing users to monitor potential safety signals in near real time.
Reporting trends show spike after MoCRA enforcement
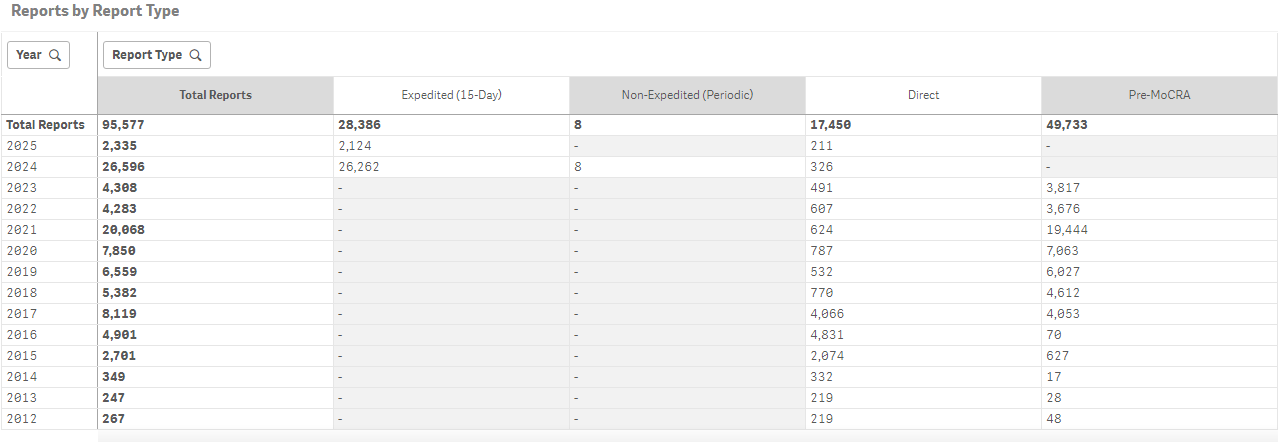
Recent dashboard data indicate a sharp increase in adverse event reporting since MoCRA took effect. Report volumes increased from just over 4,200 in 2022 to more than 28,000 in 2023, with “Expedited (15-Day)” reports making up the majority of the post-MoCRA submissions.
So far in 2025, over 2,300 reports have been filed, including more than 2,100 expedited reports, suggesting that the new reporting pathways are being actively used by industry stakeholders.
FDA emphasizes transparency, not causality
The launch of the dashboard is part of the FDA’s broader initiative to promote data transparency. “Americans are rightfully demanding greater insight into the safety and regulation of the cosmetic products they use every day,” said FDA Commissioner Marty Makary, M.D., M.P.H., in FDA’s press announcement. “This real-time dashboard is a great step in our efforts to deliver greater transparency and allow the public to help identify potential data signals.”
However, FDA cautioned that inclusion in the dashboard does not imply a confirmed link between the product and the reported adverse event. “Reports in this dashboard have not been verified by the FDA,” the agency stated in its press statement. “Their publication does not indicate that the FDA has concluded the product caused the adverse event.”
Users are encouraged to consult the agency’s published FAQs for context on how to interpret and utilize the provided data properly.
The database includes adverse event reports for various product types, such as moisturizers, shampoos, conditioners, hair dyes, and tattoos, providing stakeholders across a wide range of segments with a reason to monitor its updates regularly.
CosmeticsDesign reached out to the FDA for additional comment on the dashboard’s industry implications, but did not receive a response by the time of publication.





