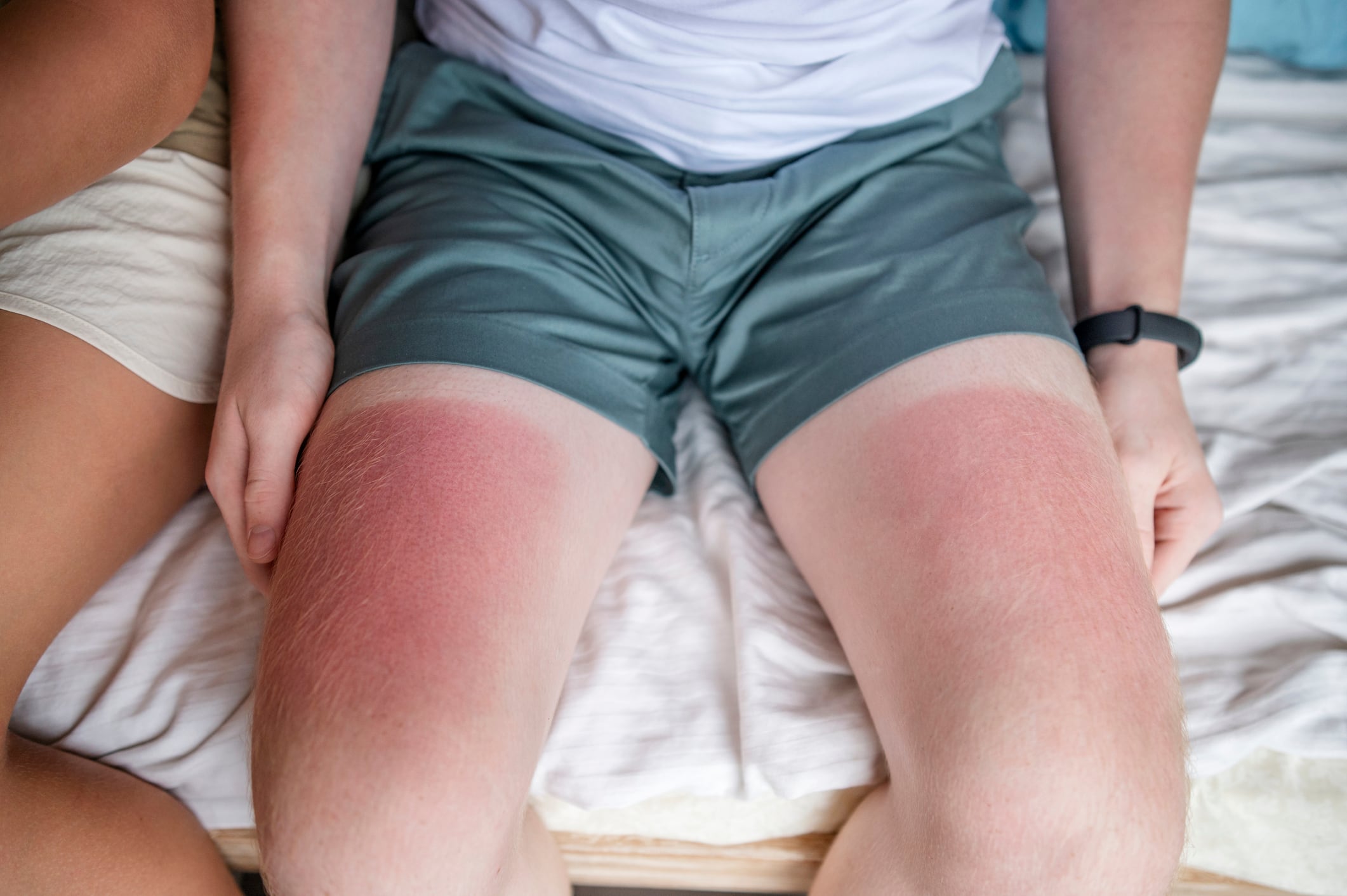
As summer approaches, the Environmental Working Group (EWG) has released its 19th annual Guide to Sunscreens, evaluating over 2,200 SPF products and identifying what it described as widespread shortcomings in sun protection efficacy and ingredient safety. According to EWG, “almost four in five, or about 80 percent, of sunscreens still offer inadequate skin protection or contain potentially harmful ingredients, or both.”
Out of 2,204 products assessed, only 498 met EWG’s strict criteria for effectiveness and safety. Among those, 63 sunscreens across 13 brands qualified for the EWG Verified mark, indicating high standards for UVA/UVB protection and ingredient transparency.
“Wearing any sunscreen is much more important and offers better sun protection for your skin than not applying anything,” said David Andrews, PhD, Acting Chief Science Officer at EWG in the organization’s press statement. “But not all sunscreens are created equal.”
Concerns regarding consumer confidence
The release has prompted strong responses from across the personal care and dermatology sectors, who cautioned against undermining public trust in sunscreen products that are tested and approved under existing regulatory frameworks.
“Sunscreen products—whether made with mineral or non-mineral UV filters—are essential, life-saving tools,” said Alexandra Kowcz, Chief Scientist and EVP of Science at the Personal Care Products Council (PCPC). “This report sows consumer confusion and poses a serious risk by undermining public trust in products that are scientifically proven, rigorously tested, and highly effective.”
Jane Yoo, MD, MPP, a dermatologist and spokesperson for the Skin Cancer Foundation, also voiced concern to CosmeticsDesign US: “At a time when social media influencers and organizations purporting to be experts have a voice, it is more critical than ever that we are delivering the same message: wear sunscreen and don’t leave home without it.”
Ingredient criticism and the SPF “booster” debate
EWG’s report places particular focus on the increased use of SPF “boosters,” including butyloctyl salicylate (BOS), which it claimed may “inflate a product’s SPF values without enhancing broad spectrum protection against the sun’s ultraviolet A or B, or UVA and UVB, rays.”
Industry experts acknowledged the need for a balance between product performance and transparency. “SPF boosters like butyloctyl salicylate enhance UVB protection and photostability, but they must be complemented with UVA filters such as avobenzone or bemotrizinol to achieve true broad-spectrum efficacy,” Carl D’Ruiz, Senior Manager of Science Advocacy at dsm-firmenich, told CosmeticsDesign US.
He stressed the importance of adhering to standardized methods like ISO 24443 or FDA UVA testing, noting that “transparent communication about the roles of SPF boosters and strict compliance with labeling regulations are essential for maintaining product integrity and consumer confidence.”
Mineral sunscreens and innovation challenges
Mineral-based sunscreens, now used in 43% of the products reviewed by the EWG, have gained traction as a safer alternative; yet, formulating these products to meet both performance and aesthetic expectations remains a challenge.
“Mineral sunscreens remain challenging due to their opacity, texture, and limited spreadability—especially for darker skin tones,” said D’Ruiz. He emphasized that limited access to UV filters in the US, only 16 FDA-approved options with only seven commonly used, makes innovation difficult compared to markets in the EU or Asia.
Dr. Yoo echoed this point from a clinical perspective. “In my practice, I constantly hear patients complain about sunscreens feeling greasy, heavy, or leaving a white cast,” she said. “There is an opportunity for brands and formulators to innovate with diverse consumers in mind.”
Toward regulatory alignment and reform
Both industry and clinical voices highlighted regulatory harmonization as a key opportunity for alignment with advocacy organizations like the EWG. “The greatest opportunity lies in modernizing regulatory science to support both safety and innovation,” said D’Ruiz. He cited the need for fast-tracking globally approved UV filters and adopting validated non-animal testing methods as possible areas for progress.
He also highlighted ongoing efforts to expand filter options in the US, noting that dsm-firmenich submitted the first FDA OTC Monograph Order Request for PARSOL Shield (bemotrizinol) last year. If approved, he said, it “could allow for the creation of sunscreen formulations that are stable and aesthetically pleasing for all skin types.”
A path forward
Despite differences in framing, many stakeholders agree that public education and access to diverse, effective sunscreens remain priorities. “Our guide helps consumers make informed choices,” said Jilly Senk, EWG’s Healthy Living Science associate, in the organization’s press statement. “Our scientists have taken the guesswork out of the search for broad spectrum sunscreens with less hazardous ingredients,” she added.
At the same time, dermatologists and formulators alike stress the importance of consistent messaging around sunscreen use. “Ensuring accessibility to sunscreen products that meet the needs of a diverse population is one of the most powerful ways to protect public health and rebuild consumer trust,” said Dr. Yoo.
As sunscreen remains one of the few preventive tools against skin cancer and photoaging, alignment between regulators, manufacturers, and advocacy groups may be crucial to drive both innovation and confidence in sun care products, especially as weather extremes continue to create an increased need for climate-adaptive skin care.